Fused Silica / Quartz
- Specially-prepared fused silica is a vital starting material used in making optical fiber for telecommunications.
- Thanks to its strength and high melting point, fused silica can be used as the envelope of halogen lamp, operating at a high envelope temperature for achieving high brightness and long life.
- It combines strength, thermal stability, and UV transparency, making it an ideal substrate for projection masks for photolithography.
- As a result of the thermal stability and composition, it is widely used in the semiconductor fabrication furnaces.
- Fused quartz has the properties to fabricate first surface mirrors for many uses (e.g.: used in telescopes). This quartz material behaves in a predictable way, and the optical fabricator can put a smooth polish onto the surface and achieve the desired figure with fewer testing iterations.
- Fused silica can be used to make many refractory shapes (including crucibles, trays, shrouds, and rollers) for many high temperature thermal processes such as steelmaking, investment casting, and glass manufacture. These refractory shapes have outstanding thermal shock resistance and are chemically inert to most elements and compounds including all acids. Translucent fused silica tubes are normally used in sheathing electric elements in room heaters, industrial furnaces and other similar applications.
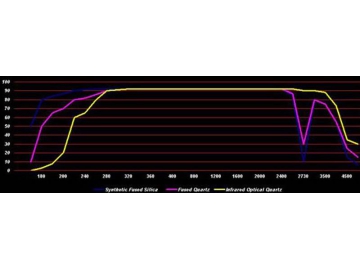
As a result of extremely low coefficient of thermal expansion, about 0.55 ppm/℃ (20-320℃), the fused quartz can undergo large, rapid temperature changes without cracking."UV grade" synthetic fused silica (trade names "HPFS", "Spectrosil" and "Suprasil"), can be transparent deeper into the ultraviolet due to its very low metallic impurity content. An optic with a thickness of 1 cm will have a transmittance of about 50% at a wavelength of 170 nm, which drops to only a few percent at 160 nm.
"IR grade" fused quartz (trade names "Infrasil", "Vitreosil IR"), being electrically fused, has more metallic impurities(limiting its UV transmittance wavelength to around 250 nm) and a much lower water content(achieving excellent infrared transmission up to 3.6 μm wavelength). Physical properties of all grades of transparent fused quartz/fused silica are near-identical.
To a large extent, manufacturing process of fused quartz and fused silica determines their water content and infrared transmission. Thanks to the combination of the hydrocarbons and oxygen fuelling the furnace forming hydroxyl [OH] within the material, flame fused material always has a higher water content. An IR grade material typically has an [OH] content of <10 parts per million.
Optical PropertiesAs a result of its high purity, the optical properties of fused quartz are better than those of other types of glass, making it ideal for use in such fields as semiconductor fabrication and laboratory equipment. With superior ultraviolet transmission, fused quartz is widely used in producing lenses and other optics for the ultraviolet spectrum.
| Wavelength | Transmission% | ||
| nm | Synthetic Fused Silica | Fused Quartz | Infrared Optical Quartz |
| 170 | 50 | 10 | 0 |
| 180 | 80 | 50 | 3 |
| 190 | 84 | 65 | 8 |
| 200 | 87 | 70 | 20 |
| 220 | 90 | 80 | 60 |
| 240 | 91 | 82 | 65 |
| 260 | 92 | 86 | 80 |
| 280 | 92 | 90 | 90 |
| 300 | 92 | 91 | 91 |
| 320 | 92 | 92 | 92 |
| 340 | 92 | 92 | 92 |
| 360 | 92 | 92 | 92 |
| 380 | 92 | 92 | 92 |
| 400-2000 | 92 | 92 | 92 |
| 2500 | 85 | 87 | 92 |
| 2730 | 10 | 30 | 90 |
| 3000 | 80 | 80 | 90 |
| 3500 | 75 | 75 | 88 |
| 4000 | 55 | 55 | 73 |
| 4500 | 15 | 25 | 35 |
| 5000 | 7 | 15 | 30 |
Quartz glass has a very low thermal coefficient of expansion (TCE) (5.0 × 10 –7/ ℃ on average), which is many times lower than that of other common materials. If 1 m3 blocks of borosilicate glass and quartz apparatus & wares were placed in a furnace and heated by 500 ℃, the volume of the borosilicate block would increase by more than 5 liters, while volume of quartz block would increase by less than one liter. Consequently, quartz glass is ideal quartz material to withstand very severe thermal shock.
Rapidly quench thin articles of quartz glass from over 1000 ℃ by plunging them into cold water without breakage is available. However, the thermal shock resistance depends on factors other than TCE such as surface condition and geometry. Varieties of used silica and fused quartz can be joined together with no added risk of thermally induced breakage due to their nearly identical TCE's.
| Technical properties | Flame Fused Quartz | Fused Silica | Electrically Fused Quartz |
| Thermal data | |||
| Softening temperature (℃) | 1660 | 1600 | 1710 |
| Annealing temperature (℃) | 1160 | 1100 | 1220 |
| Strain temperature (℃) | 1070 | 1000 | 1125 |
| Max. working temperature continuous (℃) | 1110 | 950 | 1160 |
| Max. working temperature short-term (℃) | 1250 | 1200 | 1300 |
| Mean specific heat (J/kg · K) | |||
| 0 ... 100 ℃ | 772 | 772 | 772 |
| 0 ... 500 ℃ | 964 | 964 | 964 |
| 0 ... 900 ℃ | 1052 | 1052 | 1052 |
| Heat conductivity (W/m · K) | |||
| 20 ℃ | 1.38 | 1.38 | 1.38 |
| 100 ℃ | 1.47 | 1.46 | 1.47 |
| 200 ℃ | 1.55 | 1.55 | 1.55 |
| 300 ℃ | 1.67 | 1.67 | 1.67 |
| 400 ℃ | 1.84 | 1.84 | 1.84 |
| 950 ℃ | 2.68 | 2.68 | 2.68 |
| Mean expansion coefficient (K–1) | |||
| 0 ... 100 ℃ | 5.1 × 10 –7 | 5.1 × 10 –7 | 5.1 × 10 –7 |
| 0 ... 200 ℃ | 5.8 × 10 –7 | 5.8 × 10 –7 | 5.8 × 10 –7 |
| 0 ... 300 ℃ | 5.9 × 10 –7 | 5.9 × 10 –7 | 5.9 × 10 –7 |
| 0 ... 600 ℃ | 5.4 × 10 –7 | 5.4 × 10 –7 | 5.4 × 10 –7 |
| 0 ... 900 ℃ | 4.8 × 10 –7 | 4.8 × 10 –7 | 4.8 × 10 –7 |
| – 50 ... 0 ℃ | 2.7 × 10 –7 | 2.7 × 10 –7 | 2.7 × 10 –7 |
Fused quartz material is very strong in compression, with design compressive strength is better than 1.1 x 10 9 Pa (160,000 psi). Because the inherent strength of any glass can be greatly reduced by surface flaws, tensile performance also can be greatly influenced, thus, the design tensile strength for fused quartz is more than 4.8 x 10 7 Pa (7,000 psi). In practice, a design stress of .68 x 10 7 Pa (1,000 psi) is recommended.
| Mechanical Property | Reference Value |
| Density | 2.203g/cm3 |
| Compressive Strength | >1100Mpa |
| Bending Strength | 67Mpa |
| Tensile Strength | 48.3Mpa |
| Poisson's Ratio | 0.14-0.17 |
| Elastic Modulus | 71700Mpa |
| Shearing Modulus | 31000Mpa |
| Moths Hardness | 5.3-6.5(Moths Scale) |
| Deformation Point | 1280℃ |
| Specific Heat(20-350℃) | 670J/kg.℃ |
| Thermal Conductivity(20℃) | 1.4W/m.℃ |
| Refractive Index | 1.4585 |
| Coefficient of thermal expansion | 5.5×10-7cm/cm.℃ |
| Hot work temperature | 1750-2050℃ |
| The temperature for a short time | 1300℃ |
| The temperature for a long time | 1100℃ |
| Resistivity | 7×107Ω.cm |
| Dielectric Strength | 250-400Kv/cm |
| Dielectric Constant | 3.7-3.9 |
| Dielectric absorption coefficient | < 4×104 |
| Dielectric loss coefficient | < 1×104 |
Fused quartz, also named silica, is a non-crystalline form of silicon dioxide (SiO2). High purity is essential for fused quartz, which is mostly determined by the raw material, the manufacturing method and subsequent handling procedures. The most common impurities are metals (such as Al, Na and Fe), water (present as OH groups) and chlorine, which can affect the viscosity, optical absorption and electrical properties of the quartz glass. As a result, we at BZCQ have taken special precautions at all stages of manufacture to ensure high purity. In addition, BZCQ has different purification steps to improve the quality of the quartz sand.
| NAME | UNIT X10-4% | |||||||||||||||
| Al | Fe | Ca | Mg | Yi | Cu | Mn | Ni | pb | Sn | Cr | B | K | Na | Li | OH | |
| Fused Quartz | 16 | 0.92 | 1.5 | 0.4 | 1.0 | 0.01 | 0.05 | < 0.3 | < 0.3 | < 0.3 | < 0.3 | 0.2 | 1.49 | 1.67 | < 0.3 | 400 |
| Synthetic High-pure Fused Silica | 0.37 | 0.31 | 0.27 | 0.04 | 0.03 | 0.03 | 0.01 | < 0.03 | < 0.03 | < 0.03 | < 0.03 | 0.02 | 0.5 | 0.5 | < 0.03 | 1200 |
| Infrared Optical Quartz | 35 | 1.45 | 2.68 | 1.32 | 1.06 | 0.22 | 0.07 | < 0.03 | < 0.03 | < 0.03 | < 0.03 | 0.3 | 2.2 | 3 | < 0.3 | 5 |
| Ultra-violet Optical Quartz | 3.9 | 0.4 | 3.5 | 1.2 | 0.45 | 0.1 | 0.02 | 0.06 | 0.04 | 0.02 | 0.03 | 0.1 | 0.5 | 1.5 | 0.05 | 1200 |
Fused quartz and fused silica have outstanding electrical strength that remains highly stable with temperature changes. Quartz resistivity (10 to 17th power) ohms/cc at 25 ℃ is in the order of (10 to 17th power) ohms/cc at 1000 ℃.
| Temperature ℃ | Specific Resistance ℃M | Temperature ℃ | Specific Resistance ℃M |
| 20 100 200 300 400 500 600 | 10×1018 1×1018 10×1015 0.2×1012 5×1090.3×109 60×106 | 700 800 900 1000 1100 1200 1300 | 10×106 4×106 2×106 1×106 0.7×1060.5×106 0.4×106 |
The term “super-cooled liquid” refers to the fact that quartz glass should actually be a crystalline solid rather than a liquid, which can help us to understand why quartz glass devitrifies. The thermodynamically preferred state of quartz glass is crystalline, but the high viscosity prevents the structural rearrangement necessary to achieve it, i.e., compared to the relatively fast rate of cooling that quartz glass experiences, the molecules don't have the ability to arrange themselves quickly enough. However, this constraint can be removed resulting in the glass reverting to a crystalline state under certain conditions. For example, this usually happens at elevated temperature at the presence of a contaminant (like Alkalis like sodium or potassium) that drops the viscosity by breaking up the highly connected silicon-oxygen network as well as acting as a nucleating source.
This process is also exacerbated by the atmospheres which are high in water vapor or chlorine. The growth of the devitrified layer usually starts in the surface and progresses into the material at a rate that depends exponentially on temperature. The formed crystalline material is a high temperature form of silica, known as high cristobalite. High cristobalite, with nearly the same density that glassy silica has, cannot be seen on the surface. However, upon cooling, high cristobalite changes from a cubic to a tetragonal crystal structure at about 275 ℃, which is accompanied by a large decrease in density resulting in some cracking and spalling. Refractive index differences, which result from the birefringent tetragonal crystal structure, cause the devitrified spots to turn white.
Fused QuartzFused quartz, commonly transparent, is made by melting natural high-purity quartz crystals at about 2000℃, which can use electrically heated furnace or a gas/oxygen-fuelled furnace. The naturally occurring form of fused quartz is usually referred to as Metaquartzite, which is formed under metamorphic conditions. The increasing heat makes the crystals within the quartz be fused together.
Synthetic Fused SilicaSynthetic fused silica is made from a silicon-rich chemical precursor by using a continuous flame hydrolysis process. Chemical gasification of silicon, oxidation of this gas to silicon dioxide, and thermal fusion of the resulting dust are involved in these processes, resulting in a transparent glass with a high purity and increased optical transmission in the deep ultraviolet. One common method involves adding silicon tetrachloride to a hydrogen-oxygen flame, but the use of this precursor brings some nature-unfriendly by-products such as hydrochloric acid. However, new processes (by using an alternative feedstock) have been developed to eliminate the by-products, which has also achieved a higher purity fused silica with further increased deep ultraviolet transmission.
Typical Properties of Clear Fused QuartzVery Low Coefficient of Thermal Expansion
High-Temperature Resistance
High Chemical Purity
Good Corrosion Resistance
Extensive Optical Transmission from Ultra-Violet to Infra-Red
Outstanding Electrical Insulation Qualities